Atsufumi Kawabata, Ph.D.
Professor
Address
Division
of Pharmacology & Pathophysiology
Department
of Pharmacy, Faculty of Pharmacy
Kindai
University
3-4-1 Kowakae, Higashi-Osaka 577-8502
Japan
Tel. 06-4307-3631
Fax. 06-6730-1394
Email:
kawabata@-----------------
When
you email me, please type ephar.kindai.ac.jpf after ekawabata@f.
CV

2020-the present Head of Department of Pharmacy, Faculty of Pharmacy,
Kindai University
2008-2012, 2016-2020 Head of Kindai University Graduate School of
Pharmacy
2007 (up to the present) Professor at Division of Pharmacology &
Pathophysiology, Department of Pharmacy, School of Pharmacy, Kindai University
2006 Professor at Division
of Physiology & Pathophysiology, Department of Pharmacy, School of
Pharmacy, Kindai University
2005 Professor at Division
of Physiology & Pathophysiology, School of Pharmaceutical Sciences, Kindai
University
2003 Associate Professor at
Division of Physiology & Pathophysiology, School of Pharmaceutical
Sciences, Kindai University
2001 Associate Professor at
Division of Pathophysiology & Therapeutics, School of Pharmaceutical
Sciences, Kindai University
1998 Lecturer at Division of
Pathophysiology & Therapeutics, School of Pharmaceutical Sciences, Kindai
University
1996-1997 Visiting
Scientist at Department of Pharmacology & Therapeutics, Faculty of
Medicine, University of
1994 Ph.D. (Kindai
University)
1985 Assistant at Division
of Pharmacology, School of Pharmaceutical Sciences, Kindai University
1985 M.Sci. (Kindai
University)
1983 B.Sci. (Kindai
University)
1960 Born in
Research Project
1.
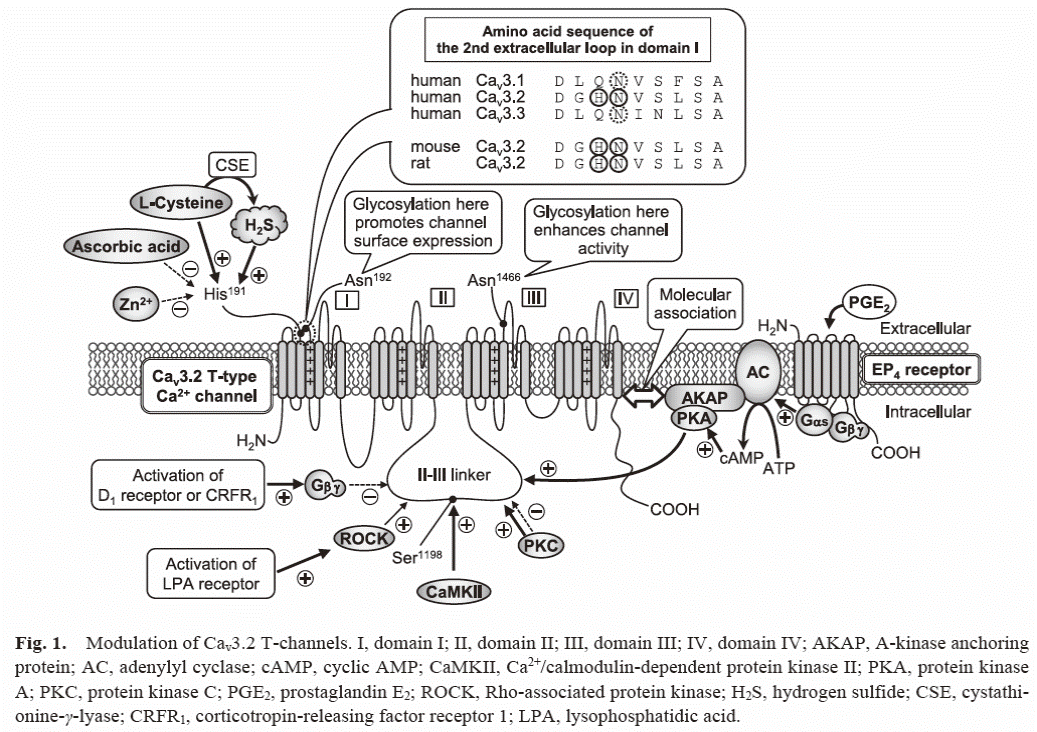
Study on T-type calcium channels
We
found that H2S, the third gasotransmitter, targets Cav3.2
T-type calcium channels, playing various the roles in the mammalian body
including processing of pain signals, neuritogenesis and modulation of
inflammatory responses. We now focus on the development of novel medicines
targeting Cav3.2, in collaboration with laboratories in other
universities and pharmaceutical companies.
Free Review Articles:
Sekiguchi and Kawabata, J Pharmacol
Sci 122, 244-250 (2013) https://www.jstage.jst.go.jp/article/jphs/122/4/122_13R05CP/_article
Schemes shown in
articles:
Tomita, S.,
Sekiguchi, F., Deguchi, T., Miyazaki, T., Ikeda, Y., Tsubota, M., Yoshida, S.,
Nguyen, H.D., Okada, T., Toyooka, N., Kawabata, A., Toxicology 413, 33-39
(2019) https://www.ncbi.nlm.nih.gov/pubmed/30552955
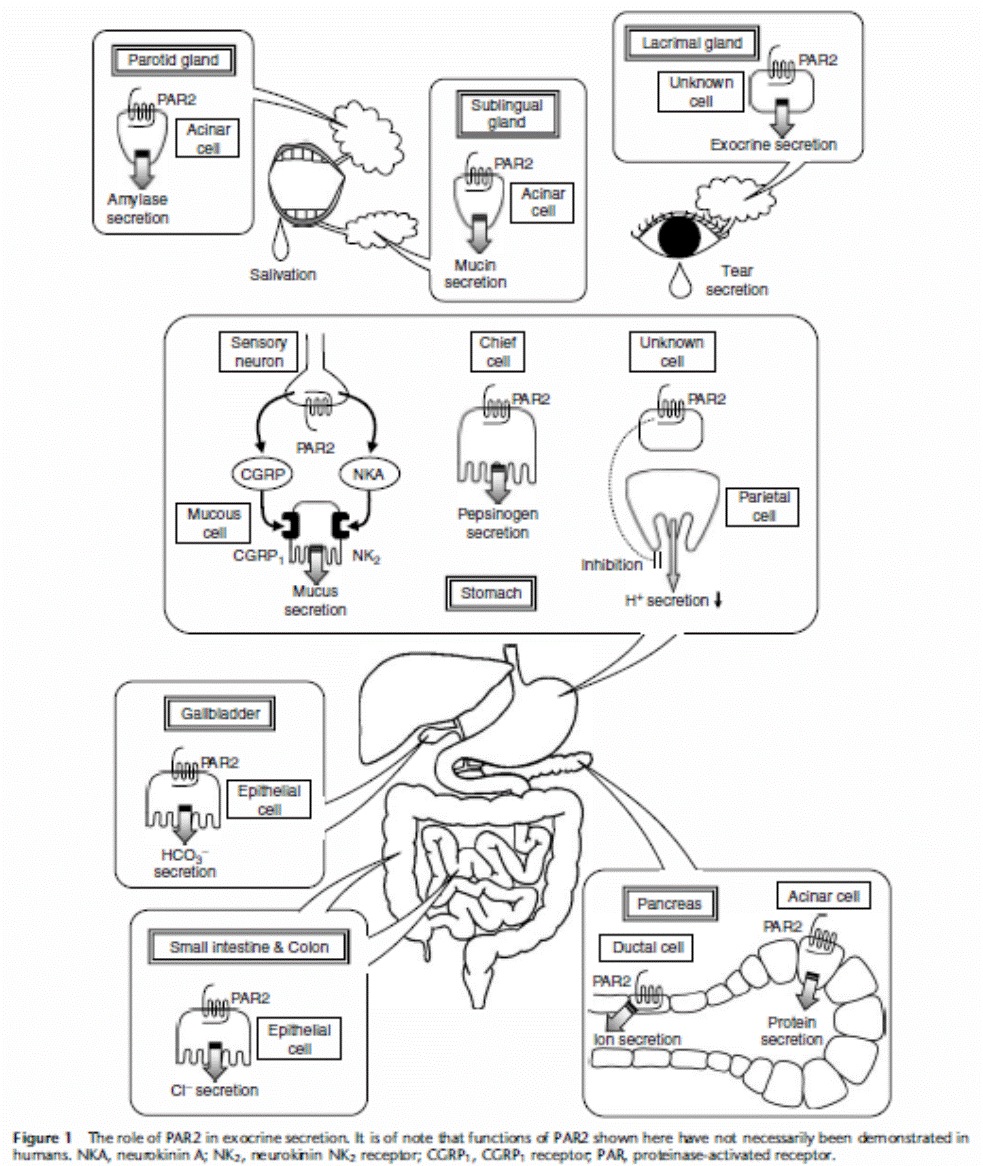
2. Study on proteinase-activated receptors (PARs)
Proteinase-activated
receptors belong to a novel family of G protein-coupled, seven trans-membrane
domain receptors that consists of four members, PARs 1, 2, 3 and 4. We have
been investigating the physiological and/or pathophysiological roles for PARs
as novel targets for development of drugs. We have reported the roles for PARs
in inflammation and in modulation of duodenal smooth muscle tone and of
salivary and pancreatic exocrine secretion. In this project, we have a lot of
collaboration with foreign universities, pharmaceutical companies and some
laboratories in our own university and others.
Free Review Articles:
Kawabata, Matsunami, Sekiguchi, Br
J Pharmacol Suppl 1, S230-S240 (2008) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2268065/
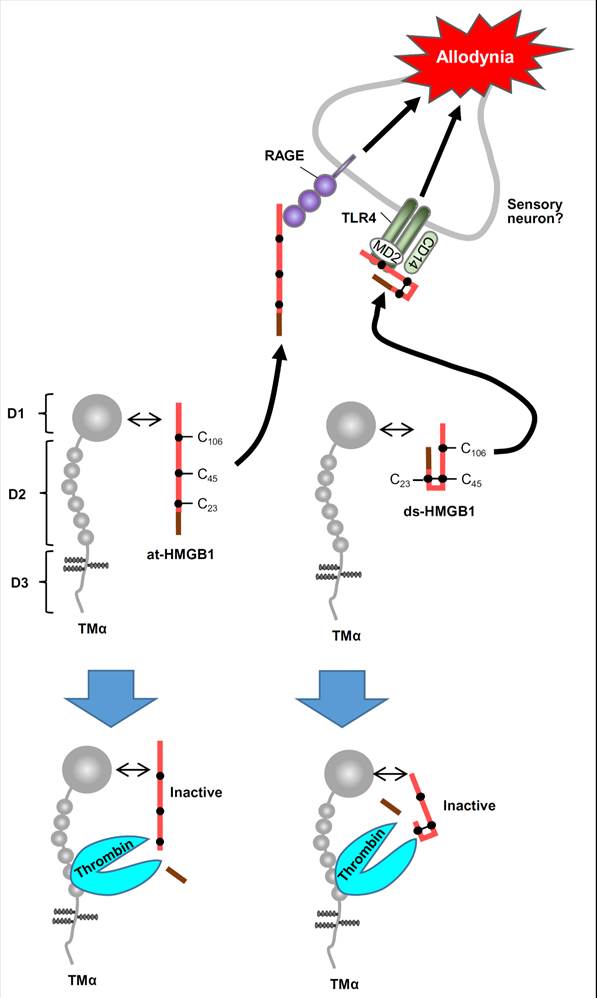
3. Study on HMGB1
We found that high mobility group
box 1 (HMGB1), one of damage associated molecular patterns (DAMPs),
participates in pain processing.
Schemes shown in
articles:
u Tsujita, Tsubota, Hayashi, Saeki,
Sekiguchi, Kawabata, J Neuroimmune Pharmacol, in press (2018) https://www.ncbi.nlm.nih.gov/pubmed/29196860
Prevention of HMGB1-induced allodynia by thrombomodulin alfa is dependent on thrombin.
u Hayashi, Tsujita, Tsubota, Saeki,
Sekiguchi, Honda, Kawabata, Biochem Biophys Res Commun 495, 634-638 (2018)
(2018) https://www.ncbi.nlm.nih.gov/pubmed/29146186
Prevention of HMGB1-induced allodynia by thrombomodulin alfa requires a minimum structure of D1-D2.
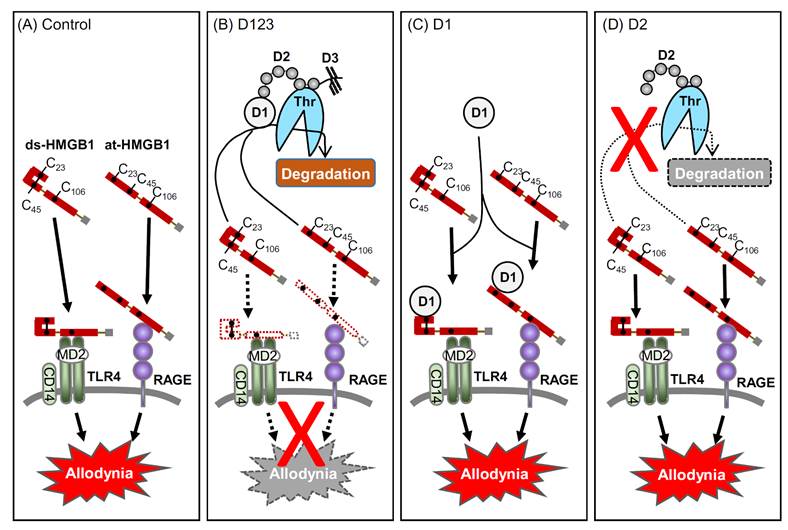
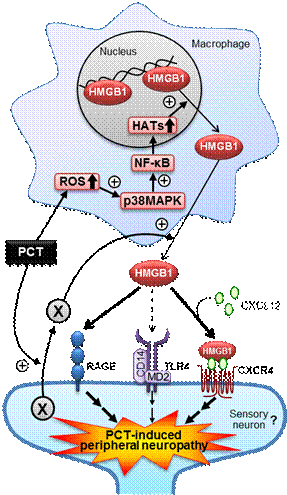
u Sekiguchi, Domoto, Nakashima, Yamasoba, Yamanishi, Tsubota, Wake, Nishibori, Kawabata, Neuropharmacology 141, 201-213 (2018) https://www.ncbi.nlm.nih.gov/pubmed/30179591
Paclitaxel-induced peripheral neuropathy involves macrophage-derived HMGB1.
4. Study on pain
modulation
Another project is about pain. We
are now studying the modulation mechanisms of pain information in the brain,
spinal cord and periphery. We wish to develop novel analgesics that can
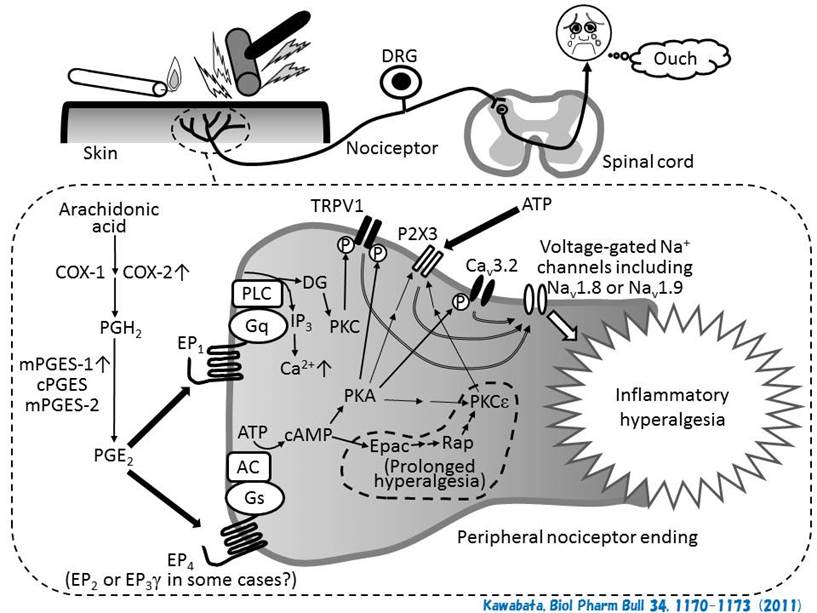
suppress intractable pain like neuropathic pain. We are now studying pain
modulation by prostaglandin E2 and HMGB1 in
addition to the above mentioned H2S, T-type calcium channels and
PARs. Our study aims at developing novel analgesics for treatment of persistent
pain including neuropathic pain.
Free Review Articles:
Kawabata, Biol Pharm Bull 34,
1170-1173 (2011) https://www.jstage.jst.go.jp/article/bpb/34/8/34_8_1170/_article
Recent publications (1998 -)
1.
Iwane, S., Nemoto, W. (co-first author), Miyamoto, T., Hayashi,
T., Tanaka, M., Uchitani, K., Muranaka, T., Fujitani, M., Koizumi, Y., Hirata,
A., Tsubota, M., Sekiguchi, F., Tan-No, K., Kawabata, A.* (2024) Clinical and
preclinical evidence that angiotensin-converting enzyme inhibitors and
angiotensin receptor blockers prevent diabetic peripheral neuropathy. Sci.
Rep., 14, 1039. PubMed
2.
Kasanami, Y., Yamamoto, T., Miyamoto, T., Matzno, S.,
Sakakibara, M., Iwaki, M., Kawabata,
A.* (2023) Characterization of potentially inappropriate medications
that need special attention in the elderly with dementia by analyzing pharmacy
claims data. Biol. Pharm. Bull., 46, 1699-1705. PubMed
3.
Tomita, S., Sekiguchi,
F., Tsubota, M., Kawabata, A.* (2023) Dietary
zinc deficiency induces Cav3.2-dependent nociceptive
hypersensitivity in mice. Biol. Pharm. Bull., 46, 1343-1346. PubMed
4.
Tomita, S., Sekiguchi,
F., Naoe, K., Shikimi, S., Kasanami, Y., Ohigashi, M., Tsubota, M., Kawabata, A.* (2023) Cav3.2-dependent
hyperalgesia/allodynia following intrathecal and intraplantar zinc chelator
administration in rodents. J. Pharmacol. Sci., 152, 86-89. PubMed
5.
Sekiguchi, F.,
Koike, N. (co-first author), Shimada, Y. (co-first author), Sugimoto, K., Masuda,
H., Nakamura, T., Yamaguchi, H., Tababe, G., Marumoto, S., Kasanami, Y., Tsubota, M., Ohkubo, T.,
Yoshida, S., Kawabata, A.*
(2023) A hydrolysate of
poly-trans-[(2-carboxyethyl)germasesquioxane]
(Ge-132) suppresses Cav3.2-dependent pain by sequestering exogenous
and endogenous sulfide. Redox Biol., 59, 102579. PubMed Outstanding paper
6.
Maeda, T., Sekiguchi,
F. (co-first author), Mitani, K., Yamagata, R., Tsubota, M., Yoshida, S., Kawabata, A.* (2023) Opioid modulation of T-type Ca2+
channel-dependent neuritogenesis/neurite outgrowth through the prostaglandin E2/EP4
receptor/protein kinase A pathway in mouse dorsal root ganglion neurons. Biochem. Biophys. Res. Commun., 639, 142-149. PubMed
7. Kasanami, Y.,
Ishikawa, C., Kino, T., Chonan, M., Toyooka, N.*, Takashima, Y., Iba, Y., Sekiguchi, F., Tsubota, M., Ohkubo, T.,
Yoshida, S., Kawase, A., Okada, T.*, Kawabata,
A.* (2022) Discovery of Pimozide Derivatives as Novel T-type Calcium
Channel Inhibitors with Little Binding Affinity to Dopamine D2
Receptors for Treatment of Somatic and Visceral Pain. Eur. J. Med. Chem., 243, 114716. PubMed
8. Miyamoto, T.,
Domoto, R. (co-first author), Sekiguchi,
F., Kawaguchi, R., Nishimura, R., Matsuno, M., Tsubota, M., Fujitani, M., Hatanaka, S., Koizumi, Y., Wang,
D., Nishibori, M., Kawabata, A.*
(2022) Development of hepatic impairment aggravates chemotherapy-induced
peripheral neuropathy following oxaliplatin treatment: Evidence from clinical
and preclinical studies. J. Pharmacol. Sci., 148 (3),
315-325. PubMed
9. Domoto, R., Sekiguchi, F. (co-first author),
Kamaguchi, R., Iemura, M., Yamanishi, H., Tsubota,
M., Wang, D., Nishibori, M., Kawabata,
A.* (2022) Role of neuron-derived ATP in paclitaxel-induced HMGB1
release from macrophages and peripheral neuropathy. J. Pharmacol. Sci., 148 (1), 156-161. PubMed
10. Ieda, S.,
Miyamoto, T. (co-first author), Hosomi, K., Takegami, M., Kawabata, A.*
(2022) Identification of remaining life expectancy less than two weeks by
CRP/albumin ratio, prognostic nutritional index, fibrosis-4 index and
albumin-bilirubin score in terminal cancer patients. J.
Palliat. Med., 25 (4), 570-576. PubMed
11. Hiramoto, S.,
Asano, H., Miyamoto, T., Takegami, M., Kawabata,
A.* (2021) Risk factors and pharmacotherapy for chemotherapy-induced
peripheral neuropathy in paclitaxel-treated female cancer survivors: A
retrospective study in Japan. PLoS One, 16 (12),
e0261473. PubMed
12. Tsubota, M., Miyazaki, T., Ikeda, Y., Hayashi, Y., Aokiba, Y., Tomita, S., Sekiguchi, F., Wang, D.,
Nishibori, M., Kawabata, A.*
(2021) Caspase-dependent HMGB1 release from macrophages participates in
peripheral neuropathy caused by bortezomib, a proteasome-inhibiting
chemotherapeutic agent, in mice. Cells, 10, 2550. PubMed
13. Hayashi, T.,
Miyamoto, T., Nagai, N., Kawabata,
A.* (2021) Development of diabetes mellitus following hormone therapy
in prostate cancer patients is associated with early progression to castration
resistance. Sci. Rep., 11, 17157. PubMed
14. Domoto, R., Sekiguchi, F. (co-first author),
Tsubota, M. (co-first
author), Kawabata, A.* (2021)
Macrophage as a peripheral pain regulator. Cells, 10, 1881. (Review) PubMed
15. Sekiguchi, F., Kawabata,
A.* (2021)
Clinical therapy targeting proteinase-activated receptors. Clin.
Neurosci. 39, 684-686. (Review)
16. Tsubota, M.,
Kawabata, A.* (2021) Physiological and pharmacological functions of
proteinase-activated receptors. Clin.
Neurosci. 39, 521-523. (Review)
17. Kawabata, A.* (2021) Subtypes of proteinase-activated receptors. Clin.
Neurosci. 39, 390-393. (Review)
18.
Miyamoto, T., Hiramoto, S. (co-first author), Kanto, Y., Tsubota, M., Fujitani, M.,
Furuyama, H., Hatanaka, S., Sekiguchi,
F., Koizumi, Y., Kawabata,
A.* (2021) Estrogen decline is a risk factor for paclitaxel-induced
peripheral neuropathy: clinical evidence supported by a preclinical study. J. Pharmacol. Sci., 146, 49-57. PubMed
19.
Tsubota,
M.,
Matsui, K. (co-first author), Fukushi, S., Okazaki, K., Sekiguchi, F., Kawabata,
A.* (2021) Effects of bepridil and pimozide, existing medicines capable
of blocking T-type Ca2+ channels, on visceral pain in mice. Biol.
Pharm. Bull., 44, 461-464. PubMed
20.
Sekiguchi,
F., Kawabata, A.* (2020) Role of
HMGB1 in chemotherapy-induced peripheral neuropathy. Int.
J. Mol. Sci., 22 (1), 367. (Review) PubMed
21.
Tsujita, R., Tsubota, M.
(co-first author), Sekiguchi, F., Kawabata, A.*
(2021) Role of high mobility group box 1 and its modulation by
thrombomodulin/thrombin axis in neuropathic and inflammatory pain. Br.
J. Pharmacol., 178, 798-812. (Review) PubMed
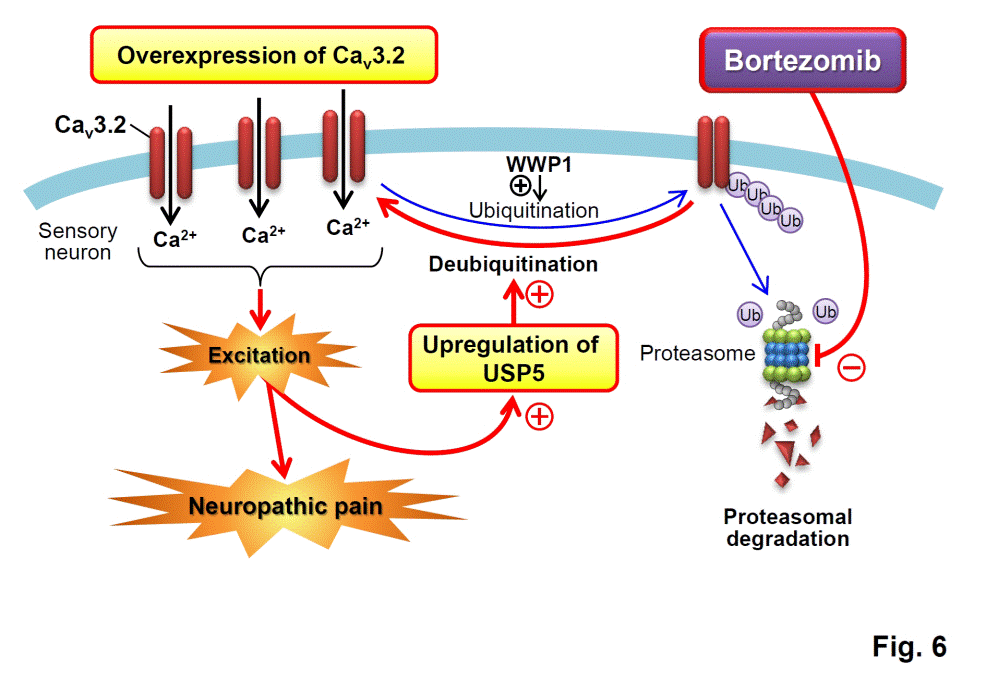
22. Tomita,
S., Sekiguchi, F. (co-first
author), Kasanami, Y., Naoe, K., Tsubota,
M., Wake, H., Nishibori, M., Kawabata,
A.* (2020) Cav3.2 overexpression in L4 dorsal root ganglion
neurons after L5 spinal nerve cutting involves Egr-1, USP5 and HMGB1 in rats:
an emerging signaling pathway for neuropathic pain. Eur.
J. Pharmacol., 888, 173587. PubMed
23. Tsubota, M., Matsui, K., Nakano, M., Kajitani, R., Ishii,
Y., Tomochika, K., Nishikawa, Y., Fukushi, S., Yamagata, A., Sekiguchi, F., Okada, T.,
Toyooka, N., Kawabata, A.*
(2020) Essential role of Cav3.2 T-type calcium channels in
butyrate-induced colonic pain and nociceptor hypersensitivity in mice. Eur.
J. Pharmacol., 887, 173576. PubMed
24. Miyamoto,
T.*, Fujitani, M., Hatanaka, S., Koizumi, Y., Kawabata, A. (2020) Use of transdermal fentanyl and elevated
gamma-glutamyl transpeptidase levels are associated with increased total daily
dose of opioid. Jpn J Pharm Palliat Care Sci, in press.
25. Hiramoto,
S., Tsubota, M. (co-first
author), Yamaguchi, K., Okazaki, K., Sakaegi, A., Toriyama, Y., Tanaka, J., Sekiguchi, F., Ishikura, H.,
Wake, H., Nishibori, M., Nguyen, H.D., Okada, T., Toyooka, N., Kawabata,A.* (2020)
Cystitis-related bladder pain involves ATP-dependent HMGB1 release from
macrophages and its downstream H2S/Cav3.2 signaling in
mice. Cells, 9, 1748. PubMed
26. Matsui,
K., Mukai, Y., Sakakura, K., Wada, K., Nakamura, T., Kawabata, A., Terakawa, N., Hayakawa, N., Kusano, K.,
Hosomi, K., Yokoyama, S., Takada, M.* (2020) Relationship between serum bepridil
concentration and corrected QT interval. Int.
J. Clin. Pharmacol. Ther., 59 (1), 63-70.
PubMed
27. Hayashi,
T., Kawaguchi, H., Eifuku, T., Matsuoka, H., Kawabata, A., Nagai, N.* (2020) Changes in percutaneous
absorption of fentanyl patches in rats treated with a sebum-like secretion. Chem.
Pharm. Bull., 68 (9), 879-884. PubMed
28. Okamoto,
H., Yoshikawa, T., Takeuchi, K., Deguchi, S., Hatakenaka, Y., Matsuoka, H., Kawabata,
A., Nagai, N.* (2020) A combination of cryopreservation and kneading
maintains the usability of Mohs past. Chem.
Pharm. Bull., 68 (6), 516-519. PubMed
29.
Irie, Y., Tsubota, M.
(co-first author), Maeda, M., Hiramoto, S., Sekiguchi, F.,
Ishikura, H., Wake, H., Nishibori, M., Kawabata, A.* (2020) HMGB1
and its membrane receptors as therapeutic targets in an intravesical substance
P-induced bladder pain syndrome mouse model. J. Pharmacol. Sci., 143 (2), 112-116. PubMed
30. Matsui,
K., Terada, Y., Tsubota, M., Sekiguchi, F., Kawabata,
A.* (2020) Tacrolimus, a calcineurin inhibitor, promotes
capsaicin-induced colonic pain in mice. J.
Pharmacol. Sci., 143 (1), 60-63. PubMed
31.
Hayashi, T., Takashina, Y., Kawaguchi, H.,
Eifuku, T., Matsuoka, H., Kawabata, A., Nagai, N.* (2019)
Evaluation of transdermal penetration in fentanyl tape using Franz diffusion cell:
changes in drug release and skin permeation under the hyperthermia. Jpn.
J. Pharmaceut. Health Care Sci., 45,
416-422.
32.
Tsubota, M., Fukuda, R., Hayashi, Y., Miyazaki, T.,
Ueda, S., Yamashita, R., Koike, N., Sekiguchi, F., Wake, H.,
Wakatsuki, S., Ujiie, Y., Araki, T., Nishibori, M., Kawabata, A.*
(2019) Role of non-macrophage cell-derived HMGB1 in oxaliplatin-induced
peripheral neuropathy and its prevention by the thrombin/thrombomodulin system
in rodents: negative impact of anticoagulants. J.
Neuroinflammation., 16 (1), 199. PubMed
33. Matsui,
K., Tsubota, M. (co-first
author), Fukushi, S., Koike, N., Masuda, H., Kasanami, Y., Miyazaki, T., Sekiguchi, F., Ohkubo, T.,
Yoshida, S., Mukai, Y., Oita, A., Takada, M., Kawabata, A.* (2019) Genetic deletion of Cav3.2
T-type calcium channels abolishes H2S-dependent somatic and visceral
pain signaling in C57BL/6 mice. J. Pharmacol. Sci., 140,
310-312. PubMed
34. Kawabata, A.*, Tsubota, M., Sekiguchi, F., Tsujita, R.
(2019) HMGB1 as a target for prevention of chemotherapy-induced peripheral
neuropathy. Nihon Yakurigaku Zasshi,
154, 236-240.
(Review) PubMed
35. Sekiguchi, F.*, Kawabata, A. (2019) Role of Cav3.2
T-type Ca2+ channels in prostate cancer cells. Nihon Yakurigaku Zasshi, 154,
97-102. (Review) PubMed
36. Tsubota, M.*, Kawabata, A. (2019) Regulation
of Cav3.2-mediated pain signals by hydrogen sulfide. Nihon Yakurigaku Zasshi, 154,
128-132. (Review) PubMed
37. Nguyen,
H.D.*, Okada, T., Sekiguchi, F.,
Tsubota, M., Nishikawa, H., Kawabata, A., Toyooka, N. (2019)
Prenylflavanones as novel T-type calcium channel blockers useful for pain
therapy. Nat. Prod. Commun., in press. (Review)
Journal HP
38. Tsubota, M., Uebo,
K., Miki, K., Sekiguchi, F.,
Ishigami, A., Kawabata, A.*
(2019) Dietary ascorbic acid restriction in GNL/SMP30-knockout mice unveils the
role of ascorbic acid in regulation of somatic and visceral pain sensitivity. Biochem. Biophys. Res. Commun., 511, 705-710. PubMed
39. Matsuda,
S., Nishikawa, H. (co-first author), Fukatsu, A., Kurokawa, Y., Tsubota, M., Sekiguchi, F., Tokuyama, S., Kawabata, A.* (2019) NNC 55-0396,
a T-type calcium channel blocker, protects against the brain injury induced by
middle cerebral artery occlusion and reperfusion in mice. J. Pharmacol. Sci., 140, 193-196. PubMed
40. Tomita,
S., Sekiguchi, F., Deguchi,
T., Miyazaki, T., Ikeda, Y., Tsubota,
M., Yoshida, S., Nguyen, H.D., Okada, T., Toyooka, N., Kawabata, A.* (2019) Critical
role of Cav3.2 T-type calcium channels in the peripheral neuropathy
induced by bortezomib, a proteasome-inhibiting chemotherapeutic agent, in mice.
Toxicology, 413, 33-39. PubMed
41. Tsubota, M., Kawabata, A.* (2019) Role of
macrophage-derived HMGB1 as an algogenic molecule/therapeutic target in
visceral pain. Pain
Res., 34, 24-30. (Review)
42. Koizumi,
Y.*, Ishiwata, S., Inoue, T., Takada, M., Kawabata,
A., Kotake, T. (2019) Investigation of the levels of ifosfamide
vaporized from powder and solution. Jpn. J. Occup. Med. Traumatol. 67, 95-99.
43. Miyamoto,
T.*, Fukuyama, H., Hatanaka, S., Fujitani, M., Koizumi, Y., Kawabata, A. (2019) The
C-reactive protein/albumin ratio is useful for predicting short-term survival
in cancer and non-cancer patients. J. Palliat. Med., 22, 532-537. PubMed
44. Sekiguchi, F.,
Domoto, R. (co-first author), Nakashima, K., Yamasoba, D., Yamanishi, H., Tsubota, M., Wake, H.,
Nishibori, M., Kawabata, A.*
(2018) Paclitaxel-induced HMGB1 release from macrophages and its implication for
peripheral neuropathy in mice: evidence for a neuroimmune cross talk. Neuropharmacology,
141, 201-213. PubMed
45. Nguyen,
H.D., Okada, T., Kitamura, S., Yamaoka, S., Horaguchi, Y., Kasanami, Y., Sekiguchi, F., Tsubota, M., Yoshida, S.,
Nishikawa, H. Kawabata, A.*,
Toyooka, N.* (2018) Design and synthesis of novel anti-hyperalgesic agents
based on 6-prenylnaringenin as the T-type calcium channel blockers. Bioorg. Med. Chem.,
26, 4410-4427. PubMed
46. Sekiguchi, F.,
Fujita, T., Deguchi, T., Yamaoka, S., Tomochika, K., Tsubota, M., Ono,
S., Horaguchi, Y., Ichii, M., Ichikawa, M., Ueno, Y., Koike, N., Tanino, T.,
Nguyen, H.D., Okada, T., Nishikawa, H., Yoshida, S., Ohkubo, T., Toyooka, N.,
Murata, K., Matsuda, H., Kawabata,
A.* (2018) Blockade of T-type calcium channels by 6-prenylnaringenin, a
hop component, alleviates neuropathic and visceral pain in mice. Neuropharmacology,
138, 232-244. PubMed
47. Sekiguchi, F., Tsubota, M., Kawabata, A.* (2018) Involvement of voltage-gated calcium
channels in inflammation and inflammatory pain. Biol. Pharm. Bull.,
41, 1127-1134. PubMed (Review)
48. Tsubota, M.,
Okawa, Y., Irie, Y., Maeda, M., Ozaki, T., Sekiguchi,
F., Ishikura, H., Kawabata,
A.* (2018) Involvement of the cystathionine-Α-lyase/Cav3.2 pathway
in substance P-induced bladder pain in the mouse, a model for nonulcerative
bladder pain syndrome. Neuropharmacology,
133, 254-263. PubMed
49. Tsubota, M.,
Ozaki, T. (co-first author), Hayashi, Y., Okawa, Y., Fujimura, A., Sekiguchi, F., Nishikawa, H., Kawabata, A.* (2018)
Prostanoid-dependent bladder pain caused by proteinase-activated receptor-2
activation in mice: Involvement of TRPV1 and T-type Ca2+ channels. J. Pharmacol. Sci.,
136, 46-49. PubMed
50. Tsujita,
R., Tsubota, M. (co-first
author), Hayashi, Y. (co-first author), Saeki, H., Sekiguchi, F., Kawabata,
A.* (2018) Role of thrombin in soluble thrombomodulin-induced suppression
of peripheral HMGB1-mediated allodynia in mice. J. Neuroimmune Pharmacol.,
13, 179-188. PubMed
51. Hayashi,
Y., Tsujita, R. (co-first author), Tsubota,
M. (co-first author), Saeki, H., Sekiguchi,
F., Honda, G., Kawabata, A.*
(2018) Human soluble thrombomodulin-induced blockade of peripheral
HMGB1-dependent allodynia in mice requires both the lectin-like and EGF-like
domains. Biochem. Biophys. Res. Commun.,
495, 634-638. PubMed
52. Ozaki,
T., Matsuoka, J., Tsubota, M.,
Tomita, S., Sekiguchi, F.,
Minami, T., Kawabata, A.*
(2018) Zinc deficiency promotes cystitis-related bladder pain by enhancing
function and expression of Cav3.2 in mice. Toxicology, 393, 102-112. PubMed
53. Ozaki,
T., Tsubota, M., Sekiguchi, F., Kawabata, A.* (2018) Involvement
of NF-ΘB in the upregulation of cystathionine-Α-lyase, a hydrogen
sulfide-forming enzyme, and bladder pain accompanying cystitis in mice. Clin. Exp. Pharmacol. Physiol.,
45, 355-361. PubMed
54. Irie,
Y., Tsubota, M. (co-first
author), Ishikura, H., Sekiguchi, F.,
Terada, Y., Tsujiuchi, T., Liu, K., Nishibori, M., Kawabata, A.* (2017) Macrophage-derived HMGB1 as a pain
mediator in the early stage of acute pancreatitis in mice: targeting RAGE and
CXCL12/CXCR4 axis. J.
Neuroimmune Pharmacol., 12, 693-707. PubMed
55. Sekiguchi, F., Kawabata, A.* (2017) Functional
regulation of ion channels by H2S and its phathological impact. Sulphuric acid and industry, 80 (5), 61-70. (Review)
56. Tsubota, M.,
Miyamoto, T., Hiruma, S., Saeki, H., Miyazaki, T., Sekiguchi, F., Funakami, Y., Kawabata, A.* (2017) Repeated cold stress reduces
cyclophosphamide-induced cystitis/bladder pain and macrophage activity in mice.
Pharmacology,
99, 286-290. PubMed
57. Tsubota, M., Kawabata, A.* (2017) Therapeutic
application of thrombomodulin alfa for visceral pain. Ulcer
Res. (Kaiyou), 44, 48-53. (Review)
58. Fukami,
K., Asano, E., Ueda, M., Sekiguchi,
F., Yoshida, S., Kawabata,
A.* (2017) High glucose induces N-linked glycosylation-mediated
functional upregulation and overexpression of Cav3.2 T-type calcium
channels in neuroendocrine-like differentiated human prostate cancer cells. J. Pharmacol. Sci.,
133, 57-60. PubMed
59. Miyamoto,
T., Funakami, Y., Kawashita, E., Tomita, S., Nomura, A., Sugimoto, N., Saeki,
H., Tsubota, M., Ichida, S., Kawabata, A.* (2017) Enhanced
hyperthermic responses to lipopolysaccharide in mice exposed to repeated cold
stress. Pharmacology,
99, 172-178. PubMed
60. Miyamoto,
T., Funakami, Y., Kawashita, E., Nomura, A., Sugimoto, N., Saeki, H., Tsubota, M., Ichida, S., Kawabata, A.* (2017) Repeated cold
stress enhances the acute restraint stress-induced hyperthermia in mice. Biol. Pharm. Bull.,
40, 1-6. PubMed
61. Terada,
Y., Tsubota, M., Sugo, H.,
Wakitani, K., Sekiguchi, F.,
Wada, K., Takada, M., Oita, A., Kawabata,
A.* (2017) Tacrolimus triggers TRPV1-dependent relapse of
pancreatitis-related pain in mice. Pharmacology, 99, 281-285. PubMed
62. Terada,
Y., Wada, K.*, Matsuda, S., Kuwahara, T., Kawabata,
A., Takada, M., Watanabe, T., Nakajima, S., Sato, T., Seguchi, O.,
Yanase, M., Fukushima, N., Nakatani, T. (2017) Circadian pharmacokinetics and
limited sampling strategy of everolimus in heat transplant patients. Int. J. Clin. Pharmacol. Ther.,
55 (1), 1-8. PubMed
63. Fukami,
K., Sekiguchi, F., Kawabata, A.* (2016) Hydrogen
sulfide and T-type Ca2+ channels in pain processing, neuronal
differentiation and neuroendocrine secretion. Pharmacology, 99, 176-203. PubMed (Review)
64. Tsubota, M., Kawabata, A.* (2016)
Nociceptors involved in pancreatitis-related pain and their importance as
therapeutic targets. Tan
to Sui, 37, 1535-1539.
(Review)
65. Nishida,
T., Tsubota, M. (co-first
author), Kawaishi, Y., Yamanishi, H., Kamitani, N., Sekiguchi, F., Ishikura, H., Liu, K., Nishibori, M., Kawabata, A.* (2016) Involvement
of high mobility group box 1 in the development and maintenance of
chemotherapy-induced peripheral neuropathy in rats. Toxicology, 365, 48-58. PubMed
66. Sekiguchi, F.,
Kawara, Y., Tsubota, M.,
Kawakami, E., Ozaki, T., Kawaishi, Y., Tomita, S., Kanaoka, D., Yoshida, S.,
Ohkubo, T., Kawabata, A.* (2016)
Therapeutic potential of RQ-00311651, a novel T-type Ca2+ channel
blocker, in distinct rodent models for neuropathic and visceral pain. Pain, 157, 1655-1665.
PubMed
67. Mitani,
K., Sekiguchi, F., Maeda, T.,
Tanaka, Y., Kawabata, A.*
(2016) The prostaglandin E2/EP4 receptor/cyclic AMP/T-type Ca2+
channel pathway mediates neuritogenesis in sensory neuron-like ND7/23 cells. J. Pharmacol. Sci.,
130, 177-180. PubMed
68. Sekiguchi, F.,
Sekimoto, T., Ogura, A., Kawabata,
A.* (2016) Endogenous hydrogen sulfide enhances cell proliferation of human
gastric cancer AGS cells. Biol.
Pharm. Bull., 39, 887-890. PubMed
69. Yamasoba,
D., Tsubota, M., Domoto, R., Sekiguchi, F., Nishikawa, H.,
Liu, K., Nishibori, M., Ishikura, H., Yamamoto, T., Taga, A., Kawabata, A.* (2016) Peripheral
HMGB1-induced hyperalgesia in mice: redox state-dependent distinct roles of
RAGE and TLR4. J.
Pharmacol. Sci., 130, 139-142. PubMed
70. Aoki,
Y., Tsubota, M., Nishimoto,
Y., Maeda, Y., Sekiguchi, F.,
Kawabata, A.* (2016)
Selective sensitization of C-fiber nociceptors by hydrogen sulfide. J. Pharmacol. Sci.,
130, 38-41. PubMed
71. Nagai,
N., Yoshioka, C., Ito, Y.*, Funakami, Y., Nishikawa, F., Kawabata, A. (2015) Intravenous administration of cilostazol
nanoparticles ameliorates acute ischemic stroke in a cerebral
ischemia/reperfusion-induced injury model. Int. J. Mol.
Sci., 16, 29329-29344. PubMed
72. Fukami,
K., Sekiguchi, F., Yasukawa,
M., Asano, E., Kasamatsu, R., Ueda, M., Yoshida, S., Kawabata, A.* (2015) Functional upregulation of the H2S/Cav3.2
channel pathway accelerates secretory function in neuroendocrine-differentiated
human prostate cancer cells. Biochem.
Pharmacol. 97, 300-309. PubMed
73. Terada,
Y., Kawabata, A.* (2015) H2S
and pain: a novel aspect for processing of somatic, visceral and neuropathic
pain signals. In Chemistry, Biochemistry and Pharmacology of
Hydrogen Sulfide, Handbook of Experimental Pharmacology, edited
by Moore, P.K. and Whiteman, M., Springer, pp . (Book Chapter)
74. Fukami,
K., Kawabata, A.* (2015)
Hydrogen sulfide and neuronal differentiation: focus on Ca2+
channels. Nitric Oxide,
46, 50-54. PubMed (Review)
75. Murakami-Nakayama,
M., Tsubota, M., Hiruma, S., Sekiguchi, F., Matsuyama, K.,
Kimura, T., Moriyama, M., Kawabata,
A.* (2015) Polaprezinc attenuates cyclophosphamide-induced cystitis and
related bladder pain in mice. J. Pharmacol. Sci.,
127, 223-228. PubMed
76. Terada,
Y., Fujimura, M., Nishimura, S., Tsubota,
M., Sekiguchi, F., Kawabata, A.* (2015) Roles of Cav3.2
and TRPA1 channels targeted by hydrogen sulfide in pancreatic nociceptive
processing in mice with or without acute pancreatitis. J. Neurosci. Res., 93, 361-369. PubMed
77. Takechi,
M.*, Wada, T., Yagi, H., Masuko, T., Kawabata,
A. (2015) Ouabain exerts cytoprotection by diminishing the
intracellular K+ concentration increase caused by distinct stressful
stimuli in human leukemic cells. J. Pharm.
Pharmacol., 67, 126-132. PubMed
78. Maeda,
Y., Sekiguchi, F., Yamanaka,
R., Sugimoto, R., Yamasoba, D., Tomita, S., Nishikawa, H., Kawabata, A.* (2015) Mechanisms for proteinase-activated
receptor 1-triggered prostaglandin E2 generation in mouse
osteoblastic MC3T3-E1 cells. Biol. Chem.,
396, 153-162. PubMed
79.
Tsubota,
M. *,
Kawabata, A. (2014) Role of
hydrogen sulfide, a gasotransmitter, in colonic pain and inflammation. Yakugaku Zasshi, 134, 1245-1252. PubMed (Review)
80. Sekiguchi, F.,
Miyamoto, Y., Kanaoka, D., Ide, H., Yoshida, S., Ohkubo, T., Kawabata, A.* (2014) Endogenous and exogenous hydrogen sulfide facilitates
T-type calcium channel currents in Cav3.2-expressing HEK293 cells. Biochem. Biophys. Res. Commun., 445, 225-229. PubMed
81. Tanaka,
J., Yamaguchi, K., Ishikura, H., Tsubota,
M., Sekiguchi, F.,
Seki, Y., Tsujiuchi, T., Murai, A., Umemura, T., Kawabata, A.* (2014) Bladder pain relief by HMGB1
neutralization and soluble thrombomodulin in mice with cyclophosphamide-induced
cystitis. Neuropharmacology, 79, 112-118. PubMed
82.
Terada, Y., Fujimura, M., Nishimura, S., Tsubota, M., Sekiguchi, F., Nishikawa, H. and
Kawabata, A.* (2013) Contribution
of TRPA1 as a downstream signal of proteinase-activated receptor-2 to
pancreatic pain. J.
Pharmacol. Sci., 123, 284-287. PubMed
83.
Tanaka, J., Seki, Y., Ishikura, H., Tsubota, M., Sekiguchi, F., Yamaguchi, K.,
Murai, A., Umemura, T. and Kawabata,
A.* (2013) Recombinant human soluble thrombomodulin prevents peripheral
HMGB1-dependent hyperalgesia in rats. Br. J. Pharmacol., 170,
1233-1241. PubMed
84.
Sekiguchi,
F.
and Kawabata, A.* (2013)
T-type calcium channels: functional regulation and implication in pain
signaling. J. Pharmacol. Sci.,
122, 244-250. PubMed
(Review)
85. Nishikawa,
H., Hayashi, H., Kubo, S., Tsubota-Matsunami,
M., Sekiguchi, F.,
and Kawabata, A.* (2013)
Inhibition by hydrogen sulfide of rabbit platelet aggregation and calcium
mobilization. Biol. Pharm. Bull., 36,
1278-1282. PubMed
86. Nakayama,
M.*, Fujiwara, M., Nakamura, T., Azuma, T., Matzno, S., Kamikonya, N., Kimura,
T., Matsuyama, K., Kawabata, A.
(2013) Stability of polaprezinc-containing oral rinse and its clinical
effectiveness against radiotherapy-induced oral mukcositis. Jpn. J. Drug Inform., 15, 133-138.
87. Nakayama,
M.*, Nakamura, T., Azuma, T., Shikata, T., Kawabata,
A., Matsuyama, K., Fujiwara, M., Kamikonya, N., Kimura, T. (2013)
Examination of a new base for the polaprezinc oral rinse. Jpn. J. Drug Inform. Jpn. J. Drug Inform., 15, 13-17.
88. Takahashi,
T., Okubo, K., Kojima, S., Nishikawa, H., Takemura, M., Tsubota-Matsunami, M., Sekiguchi,
F. and Kawabata, A.*
(2013) Antihyperalgesic effect of buprenorphine involves
nociceptin/orphanin FQ peptide receptor activation in rats with spinal nerve
injury-induced neuropathy. J. Pharmacol. Sci.,
in press. PubMed
89. Kawabata, A.* (2013)
Targeting Cav3.2 T-type calcium channels as a therapeutic strategy
for chemotherapy-induced neuropathic pain. Nihon
Yakurigaku Zasshi, 141,
81-84. PubMed (Review)
90. Sekiguchi, F., Aoki,
Y., Nakagawa, M., Kanaoka, D., Nishimoto, Y., Tsubota-Matsunami, M., Yamanaka, R., Yoshida, S. and Kawabata, A.* (2013)
AKAP-dependent sensitization of Cav3.2 channels via the EP4
receptor/cyclic AMP pathway mediates prostaglandin E2-induced
mechanical hyperalgesia. Br.
J. Pharmacol., 168, 734-745. PubMed
91.
Sekiguchi,
F.,
Matsumoto, Y., Maeda, Y., Tsubota-Matsunami,
M., Nishikawa, H. and Kawabata,
A.* (2012) Biological activity of Helicobactor pylori components in
mammalian cells: is it independent of proteinase-activated recetpros? J. Physiol. Pharmacol.,
63, 571-576. PubMed
92.
Tsubota-Matsunami,
M.,
Noguchi, Y., Okawa, Y., Sekiguchi, F.
and Kawabata, A.* (2012) Colonic
hydrogen sulfide-induced visceral pain and referred hyperalgesia involve
activation of both Cav3.2 and TRPA1 channels in mice. J. Pharmacol. Sci.,
119, 293-296. PubMed
93.
Matsunami,
M.,
Miki, T., Nishiura, K., Hayashi, Y., Okawa, Y., Nishikawa, H., Sekiguchi, F., Kubo, L., Ozaki,
T., Tsujiuchi, T. and Kawabata, A.*
(2012) Involvement of the endogenous hydrogen sulfide/Cav3.2 T-type
Ca2+ channel pathway in cystitis-related bladder pain in mice. Br. J. Pharmacol., 167,
917-928. PubMed
94.
Kawabata,
A.*
and Matsunami, M. (2012)
Roles of the hydrogen sulfide/T-type calcium channel system in somatic and
visceral pain processing. In Cell/Tissue
Injury and Cytoprotection/Organoprotection in the Gastrointestinal Tract:
Mechanisms, Prevention and Treatment, edited by Filaretova, L.P.
and Takeuchi, K., Front. Gastrointest. Res., Basel, Karger (vol 30), pp212-218. (Book Chapter)
95.
Matsunami,
M.,
Kirishi, S., Okui, T. and Kawabata,
A.* (2012) Hydrogen sulfide-induced colonic mucosal cytoprotection
involves T-type calcium channel-dependent neuronal excitation in rats. J. Physiol. Pharmacol.,
63, 61-68. PubMed
96.
Okubo, K., Matsumura, M., Kawaishi, Y.,
Aoki, Y., Matsunami, M.,
Okawa, Y., Sekiguchi, F. and Kawabata, A.* (2012) Hydrogen
sulfide-induced mechanical hyperalgesia and allodynia require activation of
both Cav3.2 and TRPA1 channels in mice. Br. J. Pharmacol., 166,
1738-1743. PubMed
97.
Okubo, K., Nakanishi, H., Matsunami, M., Shibayama, H. and
Kawabata, A.* (2012) Topical
application of disodium isostearyl 2-O-L-ascorbyl phosphate, an amphiphilic
ascorbic derivative, reduces neuropathic hyperalgesia in rats. Br. J. Pharmacol., 166,
1058-1068. PubMed
98. Kurokawa,
Y., Sekiguchi, F., Kubo, S.,
Yamasaki, Y., Matsuda, S., Okamoto, Y., Sekimoto, T., Fukatsu, A., Nishikawa,
H., Kume, T., Fukushima, N., Akaike, A. and Kawabata, A.* (2011) Involvement of ERK in NMDA
receptor-independent cortical neurotoxicity of hydrogen sulfide. Biochem. Biophys. Res. Commun., 414, 727-732. PubMed
99.
Okubo, K. and Kawabata
A.*
(2011) Roles of hydrogen sulfide in neuropathic painDPain Clinic, 32, 1464-1471. (Review)
100.
Okubo, K., Takahashi, T., Sekiguchi, F., Kanaoka, D., Matsunami, M., Ohkubo, T.,
Yamazaki, J., Fukushima, N., Yoshida, S. and Kawabata, A.* (2011) Inhibition of T-type calcium channels
and hydrogen sulfide-forming enzyme reverses paclitaxel-evoked neuropathic
hyperalgesia in rats. Neuroscience,
188, 148-156. PubMed
101.
Matsunami,
M.,
Kirishi, S., Okui, T. and Kawabata,
A.* (2011) Chelating luminal zinc mimics hydrogen sulfide-evoked
colonic pain in mice: possible involvement of T-type calcium channels. Neuroscience, 181: 257-264. PubMed
102.
Miki, T., Matsunami, M., Nakamura, S., Okada, H., Matsuya, H. and Kawabata, A.* (2011) ONO-8130, a
selective prostanoid EP1 receptor antagonist, relieves bladder pain in mice
with cyclophosphamide-induced cystitis. Pain,
152, 1373-1381. PubMed
103.
Kawabata,
A.*
(2011) Prostaglandin E2 and pain: an update. Biol. Pharm. Bull.,
34, 1170-1173. (Review) PubMed
104.
Kawabata,
A.*
(2011) Lipid Mediators and Pain Signaling: Foreword. Biol. Pharm. Bull.,
34, 1153. (Foreword) PubMed
105.
Sekiguchi,
F.,
Ohi, A., Maeda, Y., Takaoka, K., Sekimoto, T., Nishikawa, H. and Kawabata, A.* (2011) Delayed
production of arachidonic acid contributes to the delay of proteinase-activated
receptor-1 (PAR1)-triggered prostaglandin E2 release in rat gastric
epithelial RGM1 cells. J.
Cell. Biochem., 112, 909-1015.
PubMed
106.
Nishimura, S. Ishikura, H., Matsunami, M., Shinozaki, Y., Sekiguchi, F., Naruse, M.,
Kitamura, T., Akashi, R., Matsumura, K. and Kawabata, A.* (2010) The proteinase/proteinase-activated receptor-2/transient receptor
potential vanilloid-1 cascade impacts pancreatic pain in mice. Life Sci., 87, 643-650. PubMed
107.
Moriyuki, K., Sekiguchi, F., Matsubara, K., Nishikawa, H. and Kawabata, A.* (2010) Curcumin
inhibits the proteinase-activated receptor-2-triggered prostaglandin E2
production by suppressing COX-2 upregulation and Akt-dependent activation of
NF-ΘB in human lung epithelial cells. J. Pharmacol. Sci.,
114, 225-229. PubMed
108.
Fukushima, O., Nishimura, S., Matsunami, M., Aoki, Y.,
Nishikawa, H., Ishikura, H. and Kawabata,
A.* (2010) Phosphorylation of
ERK in the spinal dorsal horn following pancreatic pro-nociceptive stimuli with
proteinase-activated receptor-2 agonists and hydrogen sulfide in rats: evidence
for involvement of distinct mechanisms. J.
Neurosci. Res., 88, 3198-3205. PubMed
109.
Tarui, T., Fukami, K., Nagasawa, K.,
Yoshida, S., Sekiguchi, F.
and Kawabata, A.* (2010)
Involvement of Src kinase in T-type calcium channel-dependent neuronal
differentiation of NG108-15 cells by hydrogen sulfide. J. Neurochem., 114, 512-519. PubMed
110.
Takahashi, T., Aoki, Y., Okubo, K., Maeda,
Y., Sekiguchi, F., Mitani,
K., Nishikawa, H. and Kawabata, A.*
(2010) Upregulation of Cav3.2 T-type calcium channels targeted by endogenous
hydrogen sulfide contributes to maintenance of neuropathic pain. Pain, 150, 183-191. PubMed
111.
Takaoka, K., Sekiguchi, F., Shigi, H., Maeda, Y., Nishikawa, H. and Kawabata, A.* (2010) Opposite
effects of two thiazolidinediones, ciglitazone and troglitazone, on
proteinase-activated receptor-1-triggered prostaglandin E2 release. Toxicology, 268, 40-45. PubMed
112.
Moriyuki, K., Sekiguchi, F., Matsubara, K., Nishikawa, H. and Kawabata, A.* (2009)
PAR2-triggered prostaglandin E2 release, but not cyclooxygenase-2
upregulation, requires activation of the phosphatidylinositol 3-kinase/Akt/NFΘB
pathway in human alveolar epithelial cells. J. Pharmacol. Sci.,
111, 269-275.
PubMed
113.
Kanke, T., Kabeya, M., Kubo, S., Kondo,
S., Yasuoka, K., Tagashira, J., Ishiwata, H., Saka, M., Furuyama, T.,
Nishiyama, T., Doi, T., Hattori, Y., Kawabata,
A., Cunningham, M., Plevin, R.* (2009) Novel antagonists for
proteinase-activated receptor 2: inhibition of cellular and vascular responses
in vitro and in vivo. Br.
J. Pharmacol.,158, 361-371.
PubMed
114.
Taniguchi, E., Matsunami, M., Kimura, T., Yonezawa, D., Ishiki, T., Sekiguchi, F., Nishikawa, H.,
Maeda, Y., Ishikura, H., Kawabata, A.* (2009) Rhodanese,
but not cystathionine-Α-lyase, is associated with dextran sulfate sodium-evoked
colitis in mice: a sign of impaired colonic sulfide detoxification? Toxicology, 264, 96-103.
PubMed
115.
Kawabata,
A.*
(2009) Neuronal functions of hydrogen sulfide as a gaseous mediator. Brain21,
12, 239-245. (Review)
116.
Nishimura, S., Fukushima, O., Ishikura, H.,
Takahashi, T., Matsunami, M.,
Tsujiuchi, T., Sekiguchi, F.,
Naruse, M., Kamanaka, Y. and Kawabata,
A.* (2009) Hydrogen sulfide as
a novel mediator for pancreatic pain in rodents. Gut,
58, 762-770. PubMed Outstanding
paper
117.
Maeda, Y., Aoki, Y., Sekiguchi, F., Matsunami,
M., Takahashi, T., Nishikawa, H. and Kawabata, A.* (2009) Hyperalgesia induced by spinal and
peripheral hydrogen sulfide: evidence for involvement of Cav3.2
T-type calcium channels. Pain,
142, 127-132. PubMed
118.
Nagasawa, K., Tarui, T., Yoshida, S., Sekiguchi, F., Matsunami, M., Ohi, A., Fukami,
K., Ichida, S., Nishikawa, H. and Kawabata,
A.* (2009) Hydrogen sulfide evokes neurite outgrowth and expression of
high-voltage-activated Ca2+ currents in NG108-15 cells: involvement
of T-type Ca2+ channels. J.
Neurochem., 108, 676-684. PubMed
119.
Matsunami, M., Tarui, T., Mitani, K., Nagasawa,
K., Fukushima, O., Okubo, K., Yoshida, S., Takemura, M. and Kawabata, A.*
(2009) Luminal hydrogen sulfide plays a
pronociceptive role in mouse colon. Gut,
58, 751-761. PubMed Outstanding
paper
120.
Hirano, K.* and Kawabata, A. (2008) Basic and translational research on
proteinase-activated receptors: Preface. J. Pharmacol. Sci.,
108, 406-407. PubMed (Preface)
121.
Tanaka, Y., Sekiguchi, F.,
Hong, H. and Kawabata, A.* (2008) PAR2 triggers IL-8 release via
MEK/ERK and PI3-kinase/Akt pathways in GI epithelial cells. Biochem. Biophys. Res. Commun., 377,
622-626. PubMed
122.
Sekiguchi, F. and Kawabata,
A. (2008) Protease-activated
receptor signals. Kyukyu,
Shuchu Chiryo, 20 (9-10), 1297-1303. (Review)
123.
Kawabata, A.*, Matsunami,
M. and Sekiguchi, F. (2008) Gastrointestinal roles for
proteinase-activated receptors in health and disease. Br. J. Pharmacol., 153,
S230-S240. (Review) PubMed
124.
Nagataki, M., Moriyuki, K., Sekiguchi,
F. and Kawabata, A.* (2008) Evidence that PAR2-triggered
prostaglandin E2 (PGE2) formation involves the
ERK-cytosolic phospholipase A2-COX-1-microsomal PGE synthase-1
cascade in human lung epithelial cells. Cell
Biochem. Funct., 26, 279-282. PubMed
125.
Moriyuki, K., Nagataki, M., Sekiguchi,
F., Nishikawa, H. and Kawabata, A.* (2008) Signal
transduction for formation/release of interleukin-8 caused by a PAR2-activating
peptide in human lung epithelial cells. Regul.
Pept, 145, 42-48. PubMed
126.
Kubo, S., Kurokawa, Y., Doe, I., Masuko,
T., Sekiguchi, F. and Kawabata, A.* (2007) Hydrogen
sulfide inhibits activity of three isoforms of recombinant nitric oxide
synthase. Toxicology, 241,
92-97.
127.
Matsunami, M. and
Kawabata, A.* (2007) Is H2S considered a nociceptive
mediator? Nihon Yakurigaku Zasshi, 130, 436-437. (Review: Recent topics)
128.
Yonezawa, D., Sekiguchi, F.,
Miyamoto, M., Taniguchi, E., Honjo, M., Masuko, T., Nishikawa, H. and
Kawabata, A.* (2007) A protective role of hydrogen sulfide against
oxidative stress in rat gastric mucosal epithelium. Toxicology, 241, 11-18.
129.
Kubo, S., Doe, I., Kurokawa, Y. and Kawabata,
A.* (2007) Hydrogen sulfide causes relaxation in mouse bronchial smooth
muscle. J. Pharmacol. Sci., 104,
392-396.
130.
Kawabata, A.* (2007)
Roles for H2S in Pain Processing: Response to Cunha and Verri. Pain, 130, 302-303.
131.
Ishikura, H., Nishimura, S., Matsunami,
M., Tsujiuchi, T., Ishiki, T., Sekiguchi, F., Naruse, M.,
Nakatani, T., Kamanaka, Y. and Kawabata, A.* (2007) The
proteinase inhibitor camostat mesilate suppresses pancreatic pain in rodents. Life Sci., 80, 1999-2004.
132.
Kawabata, A.*,
Ishiki, T., Nagasawa, K., Yoshida, S., Maeda, Y., Takahashi, T., Sekiguchi,
F., Wada, T., Ichida, S. and Nishikawa, H. (2007) Hydrogen sulfide as a
novel nociceptive messenger. Pain,
132, 74-81.
133.
Kubo, S., Doe, I., Kurokawa, Y.,
Nishikawa, H. and Kawabata, A.* (2007) Direct
inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution
to dual modulation of vascular tension. Toxicology,
232, 138-146.
134.
Sekiguchi,
F.,
Saito, S., Takaoka, K., Hayashi, H., Nagataki, M., Nagasawa, K., Nishikawa, H.,
Matsui, H. and Kawabata, A.*
(2007) Mechanisms for prostaglandin E2 formation caused by
proteinase-activated receptor-1 activation in rat gastric mucosal epithelial
cells. Biochem. Pharmacol., 73,
103-114.
135.
Kubo, S., Ishiki, T., Doe, I., Sekiguchi, F., Nishikawa, H.,
Kawai, K., Matsui, H. and Kawabata,
A.* (2006) Distinct activity of
peptide mimetic intracellular ligands (pepducins) for proteinase-activated
receptor-1 in multiple cells/tissues. Ann. NY
Acad. Sci., 1091, 445-459.
136.
Matsunami, M., Sekiguchi, F. and Kawabata, A.* (2006)
Proteinase-activated receptor (PAR). Nihon Yakurigaku Zasshi, 128, 434-436.
(Review).
137.
Kawabata,
A.* (2006).
Proteinase-activated receptors and gastrointestinal functions. Nihon Yakurigaku Zasshi, 128, 82-87.
(Review).
138.
Kawabata,
A.*,
Kawao, N., Kitano, T., Matsunami, M., Satoh, R., Ishiki, T., Masuko,
T., Kanke, T. and Saito N. (2006). Colonic hyperalgesia triggered by
proteinase-activated receptor-2 in mice: involvement of endogenous bradykinin. Neurosci. Lett., 402, 167-172.
139.
Kawabata,
A.*,
Kawao, N., Hironaka, Y., Ishiki, T., Matsunami, M. and Sekiguchi, F. (2006).
Antiallodynic effect of etidronate, a bisphosphonate, in rats with
adjuvant-induced arthritis: involvement of ATP-sensitive K+
channels. Neuropharmacology, 51,
182-190.
140.
Kawabata,
A.*,
Matsunami, M., Tsutsumi, M., Ishiki, T., Fukushima, O., Sekiguchi, F., Kawao, N.,
Minami, T., Kanke, T. and Saito, N. (2006) Suppression of pancreatitis-related
allodynia/hyperalgesia by proteinase-activated receptor-2 in mice. Br. J. Pharmacol., 148, 54-60.
141.
Kawabata, A.* (2006) PAR-2 and
pain. Nihon Yakurigaku
Zasshi, 127, 133-136. (Review)
142.
Sekiguchi,
F.,
Hasegawa, N., Inoshita, K., Yonezawa, D., Inoi, N., Kanke, T., Saito, N. and Kawabata, A.* (2006) Mechanisms
for modulation of mouse gastrointestinal motility by proteinase-activated
receptor (PAR)-1 and -2 in vitro. Life Sci., 78, 950-957.
143.
Kawao, N., Nagataki, M., Nagasawa, K.,
Kubo, S., Cushing, K., Wada, T., Sekiguchi,
F., Ichida, S., Hollenberg, M.D., MacNaughton, W.K., Nishikawa, H. and Kawabata, A.* (2005) Signal transduction
for proteinase-activated receptor-2-triggered prostaglandin E2
formation in human lung epithelial cells. J. Pharmacol. Exp. Ther., 315, 576-589.
144.
Nishikawa, H. and Kawabata, A.* (2005) PAR-2 as a target for gastric mucosal
protection. Drugs of Future, 30, 793-798.
(Review)
145.
Kawabata,
A.*
(2005) Involvement of sensory nerves/TRPV1 in acute pancreatitis and. J. Gastrointestinal Res., 13, 387-392.. (Review)
146.
Nishikawa, H. and Kawabata, A.* (2005) Modulation by PARs of gastrointestinal functions. Surgery Frontier , 12, 135-143. (Review)
147.
Sekiguchi,
F.* (2005).
Development of agonists/antagonists for protease-activated receptors (PARs) and
the therapeutic possibility to gastrointestinal diseases. Yakugaku Zasshi, 125,
491-498. (Review)
148.
Kanke, T.*, Ishiwata, H., Kabeya, M.,
Saka, M., Doi, T., Hattori, Y., Kawabata,
A. and Plevin, R. (2005)
Binding of a highly potent protease-activated receptor-2 (PAR2) activating
peptide, [3H]-2-furoyl-LIGRL-NH2, to human PAR2. Br. J. Pharmacol., 145,
255-263.
149.
Kawabata, A.*,
Oono, Y., Yonezawa, D., Hiramatsu, K., Inoi, N., Sekiguchi, F., Honjo, M., Hirofuchi, M., Kanke, T. and
Ishiwata, H. (2005) 2-furoyl-LIGRL-NH2, a potent agonist for
proteinase-activated receptor-2, as a gastric mucosal cytoprotective agent. Br. J. Pharmacol., 144, 212-219.
150.
Kawabata,
A.*
and Kawao, N. (2005) Physiology and pathophysiology of proteinase-activated
receptors (PARs): PARs in the respiratory system: cellular signaling and
physiological/pathological roles. J. Pharmacol. Sci.,
97, 20-24. (Review)
151.
Kanke, T.*, Takizawa, T., Kabeya, M. and Kawabata, A. (2005) Physiology
and pathophysiology of proteinase-activated receptors (PARs):
Proteinase-activated receptor-2 (PAR-2) as a potential therapeutic target. J. Pharmacol. Sci., 97, 38-42. (Review)
152.
Nishikawa, H., Kawai, K., Tanaka, M.,
Ohtani, H., Tanaka, S., Kitagawa, C., Nishida, M., Abe, T., Araki, H. and Kawabata, A.* (2005).
Protease-activated receptor-2 (PAR-2)-related peptides induce tear secretion in
rats: involvement of PAR-2 and non-PAR-2 mechanisms. J. Pharmacol. Exp. Ther., 312, 324-331.
153.
Kimura, T., Arai,
M., Masuda, H. and Kawabata, A.*
(2004). Impact
of a pharmacist-implemented anemia management in outpatients with end-stage
renal disease in Japan. Biol. Pharm. Bull.,
27, 1831-1833
154.
Kawabata,
A.*,
Nakaya, Y., Ishiki, T., Kubo, S., Kuroda, R., Sekiguchi, F., Kawao, N., Nishikawa, H. (2004)
Receptor-activating peptides for PAR-1 and PAR-2 relax rat gastric artery via
multiple mechanisms. Life Sci., 75,
2689-2702.
155.
Sekiguchi,
F.
and Kawabata, A.* (2004)
Protease-activated receptors (PARs) as therapeutic targets: development of
agonists/antagonists and modulation of gastrointestinal functions. Drug Design Reviews, 1, 287-296. (Review)
156.
Kawabata, A.* (2004) PAR (protease-activated receptor). Seitai-No-Kagaku 55, 520-521. (Review)
157.
Kawabata,
A.*, Kubo,
S., Ishiki, T., Kawao, N., Sekiguchi,
F., Kuroda, R., Hollenberg, M.D., Kanke, T. and Saito N. (2004) Proteinase-activated receptor-2-mediated
relaxation in mouse tracheal and bronchial smooth muscle: Signal transduction
mechanisms and distinct agonist sensitivity. J.
Pharmacol. Exp. Ther., 311, 402-410.
158.
Kawabata,
A.*, Itoh,
H., Kawao, N., Kuroda, R., Sekiguchi,
F., Masuko, T., Iwata, K., Ogawa, A. (2004). Activation of trigeminal
nociceptive neurons by parotid PAR-2 activation in rats. NeuroReport,
15, 1617-1621.
159.
Sekiguchi, F., Mita, Y., Kamanaka,
Y., Kawao, N., Matsuya, H., Taga, C. and Kawabata,
A.* (2004). The potent iNOS inhibitor ONO-1714 inhibits nNOS and
exerts antinociception in rats. Neurosci. Lett., 365,
111-115.
160.
Kawabata,
A*,
Kanke, T., Yonezawa, D., Ishiki, T., Saka, M., Kabeya, M., Sekiguchi, F., Kubo, S., Kuroda, R., Iwaki, M., Katsura, K.
and Plevin, R (2004) Potent and metabolically stable agonists for
protease-activated receptor-2: Evaluation of activity in multiple assay systems
in vitro and in vivo. J.
Pharmacol. Exp. Ther. 309, 1098-1107.
161.
Kawao, N., Ikeda, H., Kitano, T., Kuroda,
R., Sekiguchi, F., Kataoka,
K., Kamanaka, Y. and Kawabata, A.*
(2004) Modulation of capsaicin-evoked visceral pain and referred hyperalgesia
by protease-activated receptors 1 and 2. J. Pharmacol. Sci.
61, 683-692.
162.
Kawabata,
A.*,
Kubo, S., Nakaya, Y., Ishiki, T., Kuroda, R., Sekiguchi, F., Kawao, N. and Nishikawa, H. (2004). Distinct
roles for protease-activated receptors 1 and 2 in vasomotor modulation in rat
superior mesenteric artery. Cardiovasc. Res.
61, 683-692.
163.
Kawabata, A.*, Nishikawa, H., Saitoh, H., Nakaya, Y.,
Hiramatsu, K., Kubo, S., Nishida, M., Kawao, N., Kuroda, R., Sekiguchi, F., Kinoshita, M.,
Kakehi, K., Arizono, N., Yamagishi, H. and Kawai, K. (2004) A protective role
of protease-activated receptor-1 in rat gastric mucosa. Gastroenterology 126, 208-219. Breakthrough
paper
164.
Kawao, N., Hiramatsu, K., Inoi, N., Kuroda, R., Nishikawa,
H., Sekiguchi, F. and Kawabata,
A.* (2003) The PAR-1-activating peptide
facilitates pepsinogen secretion in rats. Peptides 24, 1449-1451.
165.
Kamanaka, Y., Kawabata, A.*, Matsuya, H., Taga, C., Sekiguchi, F. and Kawao, N. (2003) Effect of a potent iNOS inhibitor
(ONO-1714) on acetaminophen-induced hepatotoxicity in the rat. Life Sci. 74, 793-802.
166.
Kawabata, A.*,
Nakaya, Y., Kuroda, R., Wakisaka, M., Masuko, T., Nishikawa, H. and Kawai, K.
(2003) Involvement of EDHF in the hypotension and increased gastric mucosal
blood flow caused by PAR-2 activation in rats. Br.
J. Pharmacol. 140, 247-254.
167.
Kuroda, R.*, Kawabata,
A. (2003) Pain information
pathways from the periphery to the cerebral cortex. Yakugaku Zasshi 123, 533-546 (Review) (in
Japanese)
168.
Kawabata, A.*
(2003) Gastrointestinal functions of proteinase-activated receptors. Life Sci. 74, 247-254. (Review)
169.
Kawabata, A.*
and Kawao, N. (2003) Protease-activated receptors and inflammation. BIO Clinica 18, 551-555. (Review) (in
Japanese).
170.
Nishikawa, H.
and Kawabata, A.* (2003) Modulation of gastric functions by PARs.
Drug Dev. Res. 60, 9-13. (Review)
171.
Kawabata, A.*
(2003) Physiological functions of protease-activated receptor-2. Folia Pharmacol. Japon 121,
411-420. (Review) (in Japanese).
172.
Kawabata, A.*
(2002) Multiple roles for protease-activated receptor-2 in gastric mucosa. Inflammopharmacology 10, 343-349. (Review)
173.
Kawabata, A.*
(2002) Protease-activated receptor-2 (PAR-2): roles in the nervous,
gastrointestinal and circulatory systems. Hemostasis 13, 467-476.
(Review) (in Japanese).
174.
Kawabata, A.*,
and Kuroda, R. (2002) PAR
(protease-activated receptor) as a novel target for development of gastric
mucosal cytoprotective drugs. Folia Pharmacol.
Japon. 120 (Suppl.), 85P-87P. (Review) (in Japanese).
175.
Kawabata, A.*,
Kuroda, R., Nishida, M., Nagata, N., Sakaguchi, Y., Kawao, N., Nishikawa, H.,
Arizono, N. and Kawai, K. (2002) Protease-activated receptor-2 (PAR-2) in the
pancreas and parotid gland: immunolocalization and involvement of nitric oxide
in the evoked amylase secretion. Life Sci.
71, 2435-2446.
176.
Kawabata, A.*,
Kawao, N., Itoh, H., Shimada, C., Takebe, K., Kuroda, R., Masuko, T., Kataoka,
K. and Ogawa, S. (2002) Role of N-methyl-D-aspartate receptors and the nitric
oxide pathway in nociception/hyperalgesia elicited by PAR-2 activation in mice
and rats. Neurosci. Lett. 329,
349-353.
177.
Kawabata, A.* (2002) PAR (protease-activated receptor). Seitai-No-Kagaku
53, 345. (Mini Review) (in
Japanese).
178.
Nishikawa, H.,
Kawai, K., Nishimura, S., Tanaka, S., Araki, H., Al-Ani, B., Hollenberg, M.D.,
Kuroda, R. and Kawabata, A.* (2002) Suppression by
protease-activated receptor-2 activation of gastric acid secretion in rats. Eur. J. Pharmacol. 447, 87-90.
179.
Gojyo, F.,
Sugiyo, S., Kuroda, R., Kawabata, A., Varathan, V., Shigenaga, Y.
and Takemura, M.* (2002) Effects of somatosensory cortical stimulation on
expression of c-Fos in rat medullary dorsal horn in response to
formalin-induced noxious stimulation. J.
Neurosci. Res., 68, 479-488.
180.
Kawabata, A.*
(2002) PAR-2: structure, function and relevance to human diseases of the
gastric mucosa. Expert Reviews in Molecular
Medicine (a Web journal published by Cambridge University
Press), 16 July, http://www.expertreviews.org/02004799h.htm. (Review)
181.
Kawao, N.,
Shimada, C., Itoh, H., Kuroda, R. and Kawabata, A.* (2002)
Capsazepine inhibits thermal hyperalgesia but not nociception triggered by
protease-activated receptor-2 in rats. Jpn. J.
Pharmacol., 135, 1292-1296.
182.
Kawabata, A.*,
Kawao, N., Kuroda, R., Tanaka, A. and Shimada, C. (2002) The PAR-1-activating peptide
attenuates carrageenan-induced hyperalgesia in rats. Peptides, 23, 1181-1183.
183.
Kawao, N.,
Sakaguchi, Y., Tagome, A., Kuroda, R., Nishida, S., Irimajiri, K., Nishikawa,
H., Kawai, K., Hollenberg, M.D., and Kawabata, A.* (2002)
Protease-activated receptor-2 in the rat gastric mucosa: immunolocalization and
facilitation of pepsin/pepsinogen secretion. Br.
J. Pharmacol., 135, 1292-1296.
184.
Kawabata, A.*,
Kawao, N., Kuroda, R., Itoh, H. and Nishikawa, H. (2002) Specific expression of
spinal Fos after PAR-2 stimulation in mast cell-depleted rats. NeuroReport, 13, 511-514.
185.
Kawabata, A.*,
Kinoshita, M., Kuroda, R. and Kakehi, K. (2002) Capsazepine partially inhibits
the neurally mediated gastric mucus secretion following activation of protease-activated
receptor-2. Clin. Exp. Pharmacol. Physiol.,
29, 360-361
186.
Morimoto, N.,
Nakano, M., Kinoshita, M., Kawabata, A., Morita, M., Oda, Y.,
Kuroda, R. and Kakehi, K.* (2001) Specific distribution of sialic acids
in animal tissues as examined by LC-ESI-MS after derivatization with
1,2-diamino-4,5-methylenedioxybenzene. Anal.
Chem. 73, 5422-5428.
187.
Kawabata, A.*,
Kuroda, R., Nagata, N., Kawao, N., Masuko, T., Nishikawa, H. and Kawai, K.
(2001) In vivo evidence that protease-activated receptors 1 and 2
modulate gastrointestinal transit in the mouse. Br. J. Pharmacol. 133, 1213-1218.
188.
Kawabata, A.*,
Kuroda, R., Morimoto, N., Kawao, N., Masuko, T., Kakehi, K., Kataoka, K.,
Taneda, M., Nishikawa, H. and Araki, H. (2001) Lipopolysaccharide-induced
subsensitivity of protease-activated receptor-2 in the mouse salivary glands in
vivo. Naunyn-Schmiedebergfs Arch.
Pharmacol. 364, 281-284.
189.
Kawabata, A.*, Kinoshita, M.,
Nishikawa, H., Kuroda, R., Nishida, M., Araki, H., Arizono, N., Oda, Y. and
Kakehi, K. (2001) The protease-activated receptor-2 agonist induces gastric
mucus secretion and mucosal cytoprotection. J.
Clin. Invest. 107, 1443-1450. Breakthrough
paper
190.
Kuroda, R.,
Kawao, N., Yoshimura, H., Umeda, W., Takemura, M., Shigenaga, Y. and Kawabata,
A.* (2001) Secondary somatosensory cortex stimulation facilitates the
antinociceptive effect of the NO synthase inhibitor through suppression of
spinal nociceptive neurons in the rat. Brain
Res. 903, 110-116.
191.
Kawabata, A.*,
Kuroda, R., Nakaya, Y., Kawai, K., Nishikawa,
H. and Kawao, N. (2001) Factor Xa-evoked relaxation in rat aorta: involvement
of PAR-2. Biochem. Biophys. Res. Commun. 282, 432-435.
192.
Kawabata, A.*,
Kawao, N., Kuroda, R., Tanaka, A., Itoh, H. and Nishikawa, H. (2001) Peripheral
PAR-2 triggers thermal hyperalgesia and nociceptive responses in rats. NeuroReport 12, 715-719.
193.
Kawabata, A.*,
Kuroda, R., Nakaya, Y., Kawao, N. Nishikawa, H. (2001) Ex vivo evidence
that the phosphodiesterase inhibitor IBMX attenuates the up-regulation of PAR-2
in the endotoxemic rat aorta. Thromb. Res.
101, 513-515.
194.
Kawabata,
A.* (2001) The G protein-coupled
protease receptor PAR
(protease-activated receptor) as a novel target for drug development. Yakugaku Zasshi 121,
1-7. (Review)
195.
Kataoka,
K.*, Asai, T., Taneda, M., Ueshima, S., Matsuo, O., Kuroda, R., Kawabata,
A. and Carmeliet, P. (2000) Roles of urokinase type plasminogen
activator in a brain stab wound. Brain Res.
887, 187-190.
196.
Kawabata,
A.*, Kuroda, R., Kuroki,
N., Nishikawa, H. and Kawai, K. (2000) Dual modulation by thrombin of the
motility of rat oesophageal
muscularis mucosae via two
distinct protease-activated receptors (PARs): a novel role for PAR-4 as opposed
to PAR-1. Br. J. Pharmacol. 131, 578-584.
197.
Kinoshita, M.,
Inagake, K., Kawabata, A., Kuroda, R., Oda, Y. and Kakehi, K.*
(2000) Fluorometric determination of mucin-type glycoproteins by the galactose
oxidase-peroxidase method. Anal. Biochem.
284, 87-92.
198.
Kawabata, A.,
Morimoto, N., Oda, Y., Kinoshita, M., Kuroda, R. and Kakehi, K.* (2000)
Determination of mucin in salivary glands using sialic acids as the marker by
high performance liquid chromatography with fluorometric detection. Anal. Biochem.
283, 119-121.
199.
Kawabata, A.*,
Kuroda, R., Kuroki, N., Nishikawa, H., Kawai, K. and Araki, H. (2000)
Characterization of the protease-activated receptor-1-mediated contraction and
relaxation in the rat duodenal smooth muscle. Life
Sci. 67, 2521-2530.
200.
Kawabata, A.*,
Nishikawa, H., Kuroda, R., Kawai, K. and Hollenberg, M.D. (2000) Proteinase-activated
receptor-2 (PAR-2): regulation of salivary and pancreatic exocrine secretion in
vivo in rats and mice. Br. J. Pharmacol. 129, 1808-1814.
201.
Kawabata, A.*,
Morimoto, N., Nishikawa, H., Kuroda, R., Oda, Y. and Kakehi, K. (2000)
Activation of protease-activated receptor-2 (PAR-2) triggers mucin secretion in
the rat sublingual gland. Biochem. Biophys.
Res. Commun. 270, 298-302.
202.
Kuroda, R., Kawabata,
A.*, Kawao, N., Umeda, W., Takemura, M. and Shigenaga, Y. (2000)
Somatosensory cortex stimulation-evoked analgesia in rats: potentiation by NO
synthase inhibition. Life Sci. 66, PL271-PL276.
203.
Nishikawa, H., Kawabata,
A.*, Kawai, K. and Kuroda, R. (2000) Guinea pig platelets do not
respond to GYPGKF, a protease-activated receptor-4-activating peptide: a property
distinct from human platelets. Blood Coag. Fibrinol. 11, 111-113.
204.
Kawabata, A.*
and Kuroda, R. (2000) Protease-activated receptor (PAR), a novel family of G
protein-coupled seven trans-membrane domain receptors: activation mechanisms
and physiological roles. Jpn. J. Pharmacol.
82, 171-174. (Review)
205.
Nishikawa, H., Kawabata,
A.*, Kuroda, R., Nishida, M. and Kawai, K. (2000) Characterization of
protease-activated receptors in rat peritoneal mast cells. Jpn. J.
Pharmacol. 82, 74-77.
206.
Kawabata, A.*,
Kuroda, R., Nishikawa, H. and Kawai, K. (1999) Modulation by protease-activated
receptors of the rat duodenal motility in vitro: possible mechanisms underlying
the evoked contraction and relaxation. Br. J.
Pharmacol. 128, 865-872.
207.
Kawabata, A.*,
Kuroda, R. and Hollenberg, M.D.
(1999) Physiology of protease-activated receptors (PARs): involvement of PARs
in digestive functions. Nihon Yakurigaku
Zasshi, 114 (Suppl.), 173P-179P. (Review)
(in Japanese).
208.
Kawabata, A.*,
Kuroda, R., Nishikawa, H., Asai, T., Kataoka, K. and Taneda, M. (1999)
Enhancement of vascular permeability by specific activation of
protease-activated receptor-1 in rat hindpaw: a protective role of endogenous
and exogenous nitric oxide. Br. J. Pharmacol. 126, 1856-1862.
209.
Al-Ani, B., Saifeddine,
M., Kawabata, A. and Hollenberg, M.D.* (1999) Proteinase
activated receptor 2: role of extracellular loop 2 for ligand-mediated
activation. Br. J. Pharmacol. 128, 1105-1113.
210.
Al-Ani, B.,
Saifeddine, M., Kawabata, A., Renaux, B., Mokashi, S. and
Hollenberg, M.* (1999) Proteinase-activated receptor-2 (PAR2):
development of a ligand binding assay correlating with activation of PAR2
by PAR1- and PAR2-derived peptide ligands. J.
Pharmacol. Exp. Ther. 290,
753-760.
211.
Kawabata, A.,
Saifeddine, M., Al-Ani, B., Leblond, L. and Hollenberg, M.D.* (1999)
Evaluation of proteinase-activated receptor-1 (PAR1) agonists and
antagonists using a cultured cell receptor desensitization assay: Activation of
PAR2 by PAR1-targeted ligands. J.
Pharmacol. Exp. Ther. 288, 358-370.
212.
Kawabata, A.*,
Kuroda, R., Minami, T., Kataoka, K. and Taneda, M. (1998) Increased vascular
permeability by a specific agonist of protease-activated receptor-2 in rat
hindpaw. Br. J. Pharmacol. 125,
419-422.
213.
Minami, T.*,
Okazaki, J., Kawabata, A., Kuroda, R. and Okazaki, Y. (1998)
Penetration of cisplatin into mouse brain by lipopolysaccharide. Toxicology 130, 107-113.
214.
Kawabata, A.*,
Hata, T., Kuroda, R. (1998) Cross tolerance to environmental stress and
endotoxin. Life Sci. 62,
PL327-PL333.
215.
Kawabata, A.,
Murakami, E., Iwaki, M., Ogiso, T., Suzuki, S., Mishima, M., Takada, M. and
Kakehi, K.D.* (1998) Importance of clinical activities to job
satisfaction in Japanese pharmacists.
Am. J. Health-Syst. Pharm. 55, 360-363.
216.
Minami, T.*,
Okazaki, J., Kawabata, A., Kawaki, H., Okazaki, Y. and Tohno, Y.
(1998) Roles of nitric oxide and
prostaglandins in the increased permeability of the blood-brain barrier caused
by lipopolysaccharide. Environ. Toxicol.
Pharmacol. 5, 35-41.
217.
Kawabata, A.*
and Hata, T. (1998) Disseminated intravascular coagulation (DIC)-like events
produced by environmental stress and by lipopolysaccharide: Nitric oxide as a
common key molecule. In gThe Biology of Nitric Oxide Part 6h, edited
by S. Moncada, N. Toda, H. Maeda and E.A. Higgs, pp.220, Portland Press
(London) .
218.
Kawabata, A.*
(1998) Brainstem – Endogenous analgesic substances and NO. Clinical Neuroscience 16, 775-777. (Review) (in Japanese)
